Plasma cell leukemia (PCL) is a rare plasma cell dyscrasia associated with poor outcomes. PCL can present de novo as primary PCL (pPCL) or from preceding multiple myeloma as secondary PCL (sPCL). The International Myeloma Working Group (IMWG) in 2021 updated the diagnostic criteria for pPCL using circulating plasma cells (CPC) to ≥5% instead of ≥20% as both carry similar survival. In this single center retrospective analysis, we compared clinical features and overall survival (OS) for 104 patients: with pPCL ≥5% CPC ( N = 35), sPCL 5-19% CPC (sPCL-A) ( N = 11), sPCL ≥20% CPC (sPCL-B) ( N = 38), and spillover group 1-4% CPC regardless of newly diagnosed or secondary status ( N = 20).
Mean ages were 57 - 59 years old for all groups (range: 25 - 84) with no difference in age between groups ( p = 0.90). There was a slight predominance of females (54.8%) over males and blacks (53.9%) over non-blacks. More patients with pPCL were black (65.7%) compared to sPCL and spillover groups, although this difference was not statistically significant ( p = 0.35). pPCL patients presented with the highest mean WBC at diagnosis (23.4 x10 9/L) compared to sPCL-A (5.5 x10 9/L), sPCL-B (12.2 x10 9/L), and spillover (7.2 x10 9/L) groups ( p <0.001). Patients in all groups were anemic (hemoglobin 7.8 - 9.3 g/dL), thrombocytopenic (platelets 60.2 - 101.2 x 10 9/L), and had abnormal renal function (creatinine 1.4 - 2.7 mg/dL), elevated LDH (918.7 - 1515.5 units/L, highest in sPCL-A), and elevated uric acid levels (5.9 - 8.2 mg/dL). sPCL patients and spillover group had more lytic lesions on skeletal survey ( p = 0.009) and positron emission tomography (PET) ( p = 0.02) compared to patients with pPCL. sPCL-A patients had higher incidences of lymphadenopathy ( N = 5/11, 62.5%, p = 0.02) and central nervous system invasion ( N = 4/11, 36.4%, p = 0.02) compared to other groups. There was no difference in incidence of extramedullary disease ( p = 0.29) between groups.
Over 90% of patients with PCL had high-risk cytogenetics with 63% carrying a complex karyotype. Hypodiploidy was seen more often in patients with pPCL and sPCL-B compared to sPCL-A and spillover ( p = 0.005). There was no difference between groups in incidence of deletion 17p ( p = 0.53). All patients benefited in terms of OS from daratumumab (HR 0.44, 95% CI 0.25 - 0.76; p = 0.004), IMiDs (HR 0.13, 95% CI 0.08 - 0.23; p <0.001), PIs (HR 0.43, 95% CI 0.25 - 0.75; p = 0.003), chemotherapy (HR 0.56, 95% CI 0.32 - 0.98; p = 0.04), and autologous hematopoietic stem cell transplantation (HR 0.33, 95% CI 0.16 - 0.71; p = 0.004). DVT-PACE type regimens (dexamethasone-bortezomib-thalidomide-cisplatin-doxorubicin-cyclophosphamide-etoposide) are used for pPCL and may be less effective in sPCL and spillover patients who have been heavily pretreated. We noted no benefit in OS for all patients including pPCL, sPCL-A, sPCL-B, and spillover groups who received DVT-PACE (HR 0.97, 95% CI 0.62 - 1.50, p = 0.88). The lack of OS benefit in pPCL patients receiving DVT-PACE persisted on multivariate analysis when adjusting for PCL group alone (HR 0.79, 95% CI 0.50 -1.26; p = 0.33) and PCL group, age, platelet count, and presence of high-risk cytogenetics (HR 1.11, 95% CI 0.63 - 1.94; p = 0.72).
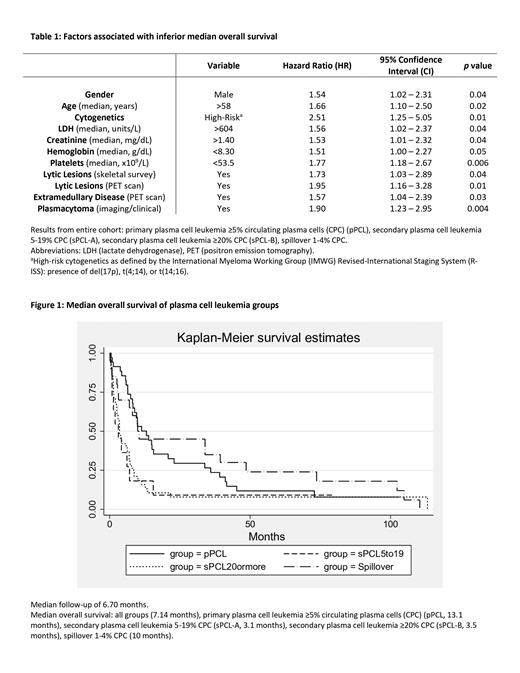
After median follow-up of 6.70 months, median OS was 7.14 months for the entire cohort with 92.3% ( N = 96) deceased. On univariate analysis, male sex, age >58 years old, high-risk cytogenetics, LDH >604 units/L, creatinine >1.40 mg/dL, hemoglobin <8.3 g/dL, platelets <53.5 x10 9/L, lytic lesions on skeletal survey and on PET, extramedullary disease on PET, and plasmacytoma were associated with inferior median OS (Table 1). pPCL had higher median OS (13.1 months) compared to sPCL-A (3.1 months, p = 0.03) and sPCL-B (3.5 months, p = 0.003) (Figure 1). There was no statistically significant difference in median OS between pPCL (13.1 months) and spillover patients (10 months) ( p = 0.48) as well as sPCL-A (3.1 months) and sPCL-B (3.5 months) ( p = 0.80). Although the spillover group was associated with a trend towards better OS compared to sPCL-A and sPCL-B groups, this was not statistically significant, which may be due to low N which is a limitation of the study. Further research is required to better understand the clinical features, treatment responses, and outcomes of pPCL, sPCL, and spillover group (1-4% CPC).
Disclosures
Badros:Janssen: Research Funding; GSK: Research Funding; BMS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal